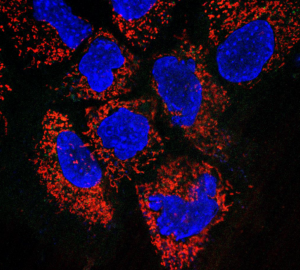
Mitochondrial labeling in salivary gland cells. CellLight® Mitochondria-RFP, BacMam 2.0, (ThermoFisher) was used transfect human salivary gland cells to label them with RFP. The image was captured 24h post transfection. Mitochondria are red and nuclei are stained in blue.
Mitochondrial damage and activation of innate immunity in salivary gland cells. Recent literature shows that increased oxidative stress causes mitochondrial dysfunction, leading to the release of mitochondrial DNA (mtDNA) into the cytosol. This mtDNA is recognized as a danger signal, and it induces type I IFN and pro-inflammatory cytokines through the cyclic GMP-AMP synthase (cGAS)-Stimulator of interferon genes (STING) protein-interferon regulatory factor 3 (IRF3) axis. Furthermore, the activation of the cGAS-STING-IRF3 axis also causes cellular senescence, contributing to the adverse effects of aging. Despite the evidence for oxidative stress and elevated type I IFN response, the role of mtDNA in the activation of innate immunity in Sjogren’s disease is not known. Therefore, our laboratory has generated in vitro and in vivo genetic models for inducing mitochondrial damage in mouse salivary glands. These model systems are being used to investigate the role of oxidative stress and excessive activation of innate immunity in the development of Sjogren’s disease.
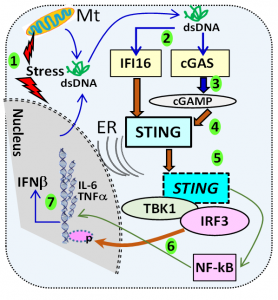 Schematic representation of STING activation. (1) Cellular stress can cause the leakage of nuclear and mitochondrial DNA into the cytosol (2) recognized by cytosolic DNA sensors like cGAS. (3) The engagement of cGAS leads to the production of the di-nucleotide cGAMP, (4) which binds STING. (5) STING translocates from the ER binds the serine-threonine kinase TBK1, which then phosphorylates IRF3. (6) Phosphorylated IRF3 enters the nucleus and initiates transcription of IFNb (7). In addition, STING also activates NF-kB and induces transcription of IL-6 and TNFa. The SS-associated protein, IFI16 can also activate STING through a non-canonical pathway involving p53 and TRAF6.
Schematic representation of STING activation. (1) Cellular stress can cause the leakage of nuclear and mitochondrial DNA into the cytosol (2) recognized by cytosolic DNA sensors like cGAS. (3) The engagement of cGAS leads to the production of the di-nucleotide cGAMP, (4) which binds STING. (5) STING translocates from the ER binds the serine-threonine kinase TBK1, which then phosphorylates IRF3. (6) Phosphorylated IRF3 enters the nucleus and initiates transcription of IFNb (7). In addition, STING also activates NF-kB and induces transcription of IL-6 and TNFa. The SS-associated protein, IFI16 can also activate STING through a non-canonical pathway involving p53 and TRAF6.